Breakthrough in drinking water softening
- info7643583
- 17 jun 2025
- 2 minuten om te lezen
Breakthrough in drinking water softening – incredibly proud of Sergěj Seepma
Today it’s official. Our two latest papers have been published in Water Research and are now freely accessible via open access.
I am incredibly proud of Sergěj Seepma, who has done the real heavy lifting in this work.
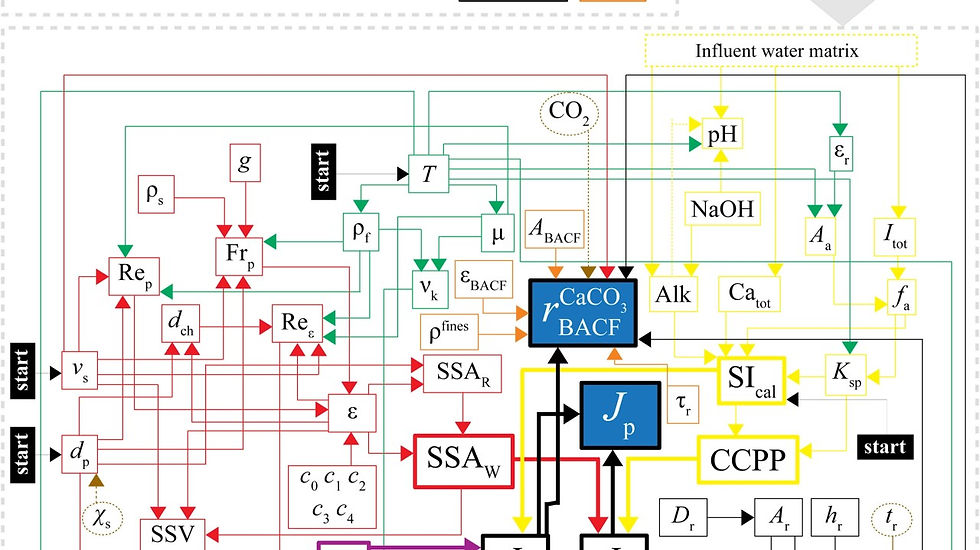
What Sergěj has achieved is exceptional. The highly complex interplay between hydrodynamics, thermodynamics, mass transfer kinetics and nucleation in pellet softening has now been captured in a fully mechanistic model. In addition, the relationship between particle behaviour, fluid flow, reactor geometry and the composition of the water itself has been clearly quantified and brought together.
To put it more simply, we now understand not just what happens inside a softening reactor, but why it happens. Whether calcium deposits correctly onto pellets or forms unwanted fine particles depends on mixing, flow dynamics and the degree of saturation. These are a combination of chemical reactions and physical processes, and they are now described together in one predictive model.
For the first time, we can distinguish and quantify the difference between crystal growth on pellets and the formation of undesirable particles in the water itself. This allows us to design and operate softening reactors based on physical and chemical principles rather than relying on experience and trial-and-error. The result is more stable, efficient and predictable operation.
This comes at the perfect moment. Sodium hydroxide, which is essential in the process, is increasingly being produced sustainably. Our earlier work already showed that pellet softening has a very low climate footprint (Beeftink et al., Journal of Cleaner Production, 2021). Thanks to this new development in sustainable chemical sourcing, the process becomes even more climate-friendly while remaining simple and effective.
These insights offer a completely new perspective on how pellet softening works in drinking water treatment. They are also highly relevant to broader applications such as Crystallactor technology and other crystallisation-based processes. This is a major step forward in designing more robust and future-proof treatment systems.
Thank you to all colleagues involved at Waternet, Utrecht University, Delft University of Technology and Queen Mary University of London. And a special thanks to the team on site. Your input, observations and persistence made all the difference.
The publications can be accessed here:
Mechanistic model advancements for optimal calcium removal in water treatment
Operational control strategy on optimal calcium removal in drinking water treatment
Earlier related publication on the carbon footprint of softening:
Beeftink, Hofs, Kramer, Odegard, van der Wal – Journal of Cleaner Production (2021)
and
Schetters, van der Hoek, Kramer, Kors, Palmen, Hofs, Koppers, 2015. Circular economy in drinking water treatment: reuse of ground pellets as seeding material in the pellet-softening process. Water Science and Technology
#chemistry #hydraulics #multiphaseflow #waterresearch #drinkingwater #softening #crystallisation #sustainability #engineering #crystallactor #futureproof #processdesign #waternet #teamscience



Opmerkingen